| Sulfur is yellow in its element state. |
Sulfur
Sulfur (in traditional lay Commonwealth English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. SULFUR-36 isotope is used for production of Sulphur-37 (S-37 isotope, 37S isotope, Sulfur-37) and Sulphur-38 (S-38 isotope, 38S isotope, Sulfur-38) radionuclide (radioisotope); Sulfur-36 isotope is available to order from BuyIsotope.com in Sulfur-36 elemental (S) chemical form. Atomic Number: 16: Atomic Radius: 180 pm (Van der Waals) Atomic Symbol: S: Melting Point: 115.21 °C: Atomic Weight: 32.06. Sulfur is found in meteorites.
Uses of Sulfur - Sulphur is a chemical element with symbol S and atomic number 16. Know the Uses of Sulphur, Chemical Properties of Sulphur, Atomic Mass, Melting Point and more at BYJU'S.
Sulfur Atomic Number Of Protons
| Atomic Number: | 16 | Atomic Radius: | 180 pm (Van der Waals) |
| Atomic Symbol: | S | Melting Point: | 115.21 °C |
| Atomic Weight: | 32.06 | Boiling Point: | 444.60 °C |
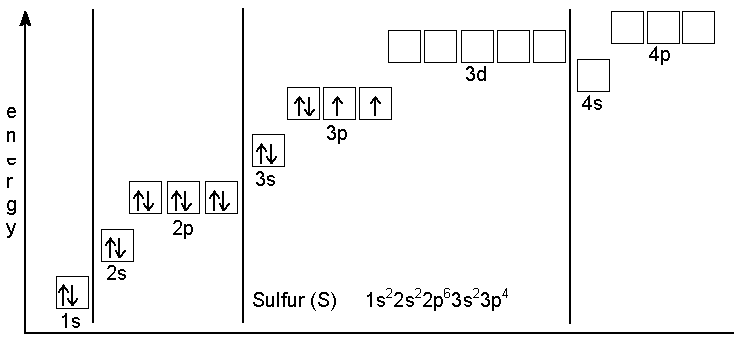
| Electron Configuration: | [Ne]3s23p4 | Oxidation States: | 6, 5, 4, 3, 2, 1, −1, −2 (a strongly acidic oxide) |
History
Known to the ancients; referred to in Genesis as brimstone.
Sources
Sulfur is found in meteorites. R.W. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit.
Sulfur occurs native in the vicinity of volcanos and hot springs. It is widely distributed in nature as iron pyrites, galena, sphalerite, cinnabar, stibnite, gypsum, epsom salts, celestite, barite, etc.
Production
Sulfur is commercially recovered from wells sunk into the salt domes along the Gulf Coast of the U.S. Using the Frasch process heated water is forced into the wells to melt the sulfur, which is then brought to the surface.
Sulfur also occurs in natural gas and petroleum crudes and must be removed from these products. Formerly this was done chemically, which wasted the sulfur; new processes now permit recovery. Large amounts of sulfur are being recovered from Alberta gas fields.
Properties
Sulfur is pale yellow, odorless, brittle solid, which is insoluble in water but soluble in carbon disulfide. In every state, whether gas, liquid or solid, elemental sulfur occurs in more than one allotropic form or modification; these present a confusing multitude of forms whose relations are not yet fully understood.
Smart technologies ulc driver download for windows 10. In 1975, University of Pennsylvania scientists reported synthesis of polymeric sulfur nitride, which has the properties of a metal, although it contains no metal atoms. The material has unusual optical and electrical properties.

High-purity sulfur is commercially available in purities of 99.999+%.
Amorphous or 'plastic' sulfur is obtained by fast cooling of the crystalline form. X-ray studies indicate that amorphous sulfur may have a helical structure with eight atoms per spiral. Crystalline sulfur seems to be made of rings, each containing eight sulfur atoms, which fit together to give a normal X-ray pattern.
Isotopes
Eleven isotopes of sulfur exist. None of the four isotopes that are found in nature are radioactive. A finely divided form of sulfur, known as flowers of sulfur, is obtained by sublimation.
Compounds
Organic compounds containing sulfur are very important. Calcium sulfur, ammonium sulfate, carbon disulfide, sulfur dioxide, and hydrogen sulfide are but a few of the many important compounds of sulfur.
Uses
Sulphur Atomic Number Electron Configuration
Sulfur is a component of black gunpowder, and is used in the vulcanization of natural rubber and a fungicide. It is also used extensively in making phosphatic fertilizers. A tremendous tonnage is used to produce sulfuric acid, the most important manufactured chemical.
It is used to make sulfite paper and other papers, to fumigate, and to bleach dried fruits. The element is a good insulator.

Sulfur is essential to life. It is a minor constituent of fats, body fluids, and skeletal minerals.
Sulphur Atomic No
Handling
Sulphur Atomic Symbol
Carbon disulfide, hydrogen sulfide, and sulfur dioxide should be handled carefully. Hydrogen sulfide in small concentrations can be metabolized, but in higher concentrations it quickly can cause death by respiratory paralysis.
Sulphur Atomic Weight
It quickly deadens the sense of smell. Sulfur dioxide is a dangerous component in atmospheric air pollution. Drivers scm sound cards & media devices.

